The Race To a Vaccine
A small bottle of a COVID-19 vaccine.(Image Credit: Sakchai Lalit / Associated Press) (Courtesy of the LA Times)
People are anticipating the end of COVID-19, and, therefore, quarantine, but the extermination of the coronavirus can only be reached by one of three ways: the gradual phasing out of the virus, a vaccine, or a cure. The most practical of these methods is the vaccine. However, most vaccines take years to make and need to go through a rigorous process of research and testing before it can be given to people. There are already many vaccines for the coronavirus in advanced stages of testing, which could be in use as soon as next year.
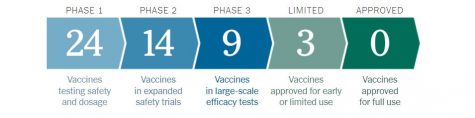
There are currently at least 37 vaccines in clinical trials being tested on humans, and at least 91 preclinical vaccines that are under analysis on the effects they have on animals.
There are many different types of vaccines that are being developed. These include: genetic vaccines, which use one or more of the coronavirus’s own genes to provide an immune response; viral vector vaccines, which use a virus to deliver coronavirus genes into cells that therefore make viral proteins (the virus cannot replicate), provoking an immune response; protein based vaccines, which use a coronavirus protein or a protein fragment to provoke an immune response; whole-virus vaccines, which use weakened or inactivated viruses to provoke an immune response; and repurposed vaccines, which are vaccines that are already in use for other diseases that may also protect against COVID-19.
Many organizations are working on vaccines, including Moderna, in partnership with National Institutes of Health, German company BioNTech in collaboration with New York-based Pfizer and Chinese drug maker Fosun Pharma, and British-Swedish company AstraZeneca in cooperation with the University of Oxford.

Additionally, there are 3 vaccines from Chinese and Russian companies that have been approved for early or limited use. However, these vaccines were approved before receiving the results of their Phase 3 trials. Some experts say that the rushed process poses serious risks.
On September 8, AstraZeneca halted its vaccine trials because an enrollee in its Phase 2/3 trial had developed symptoms of a condition called transverse myelitis, which causes inflammation of the spinal cord.
Despite setbacks like this, many companies are working to get a vaccine out as soon as possible. A vaccine will speed up the process of eliminating COVID-19, and allow the world to go back to normal.
To learn more about the vaccines that are being developed, visit the coronavirus vaccine tracker on the New York Times website.
Source
https://www.nytimes.com/2020/09/09/world/covid-coronavirus.html
